
60 Degrees Pharmaceuticals Inc
$1.08 +0.0050 +0.47% 114.9K
Developing therapeutic innovations that can significantly improve and extend people’s lives

Analyst Report
Q2 FY24 impacted by non-cash charge. Babesiosis trial on track, with timeline somewhat extended. Re-setting PT to $5.50.
Company Overview
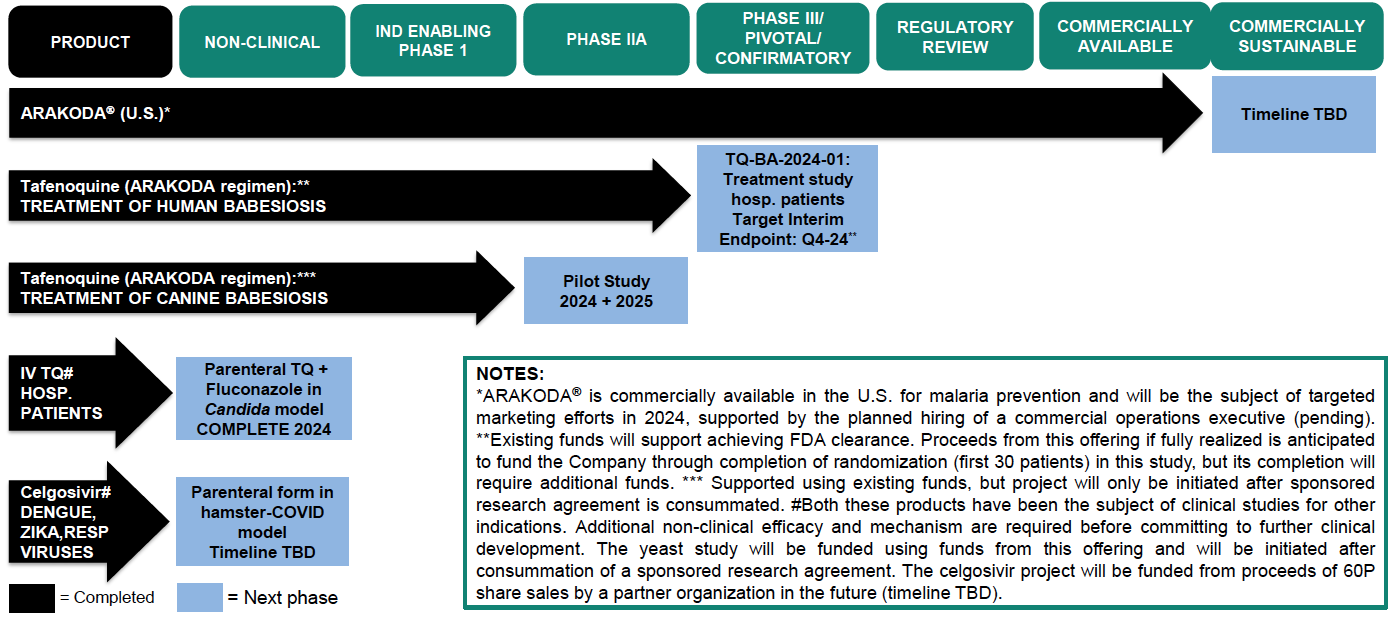
60 Degrees Pharmaceuticals (60P) is a growth-oriented biotechnology company with a goal of using cutting-edge biological science and applied research to further develop and commercialize new therapies for the prevention and treatment of infectious diseases. 60P has successfully achieved regulatory approval of ARAKODA® (tafenoquine), a malaria preventative treatment that has been on the market since late 2019. Currently, 60P’s pipeline includes development programs for Tafenoquine and Celgosivir targeting fungal, tick-borne, and other viral diseases.
Value Proposition
60P is addressing the unmet medical need associated with infectious diseases through the development and commercialization of new small molecule therapeutics. By focusing on synthetic drugs (made by chemists in labs, excluding biologics) with good safety profiles based on prior clinical studies, 60P believes it has a cost-effective path to new indications that capitalizes on existing research to reduce costs and risk. 60P is expanding its commercialization efforts related to ARAKODA (tafenoquine), an antimalarial indicated for prophylaxis of malaria in patients 18 years and older and approved by the FDA in 2018. In Q2 2023, sales of ARAKODA increased by 150% relative to the same period in 2022, at an accelerating growth rate.
60P is implementing clinical research programs to evaluate the utility of the ARAKODA regimen of tafenoquine for non-malaria disease indications, with an upcoming planned pivotal study of tafenoquine in hospitalized babesiosis patients. 60P anticipates initiating patient enrollment for this trial in mid-2024. According to Company estimates, 47,000 cases of babesiosis (infections caused by red blood cell parasites similar to malaria that are transmitted by deer tick bites) occur in the United States each year, and the incidence rate is increasing. Estimates are that 10% of Lyme disease patients are co-infected with babesiosis. 60P is also testing the viability of another product (Celgosivir) to determine whether to advance it into further clinical development and may seek to develop and license other molecules in the future.
Anticipated Future Milestones
(Pivotal Babesiosis Study)
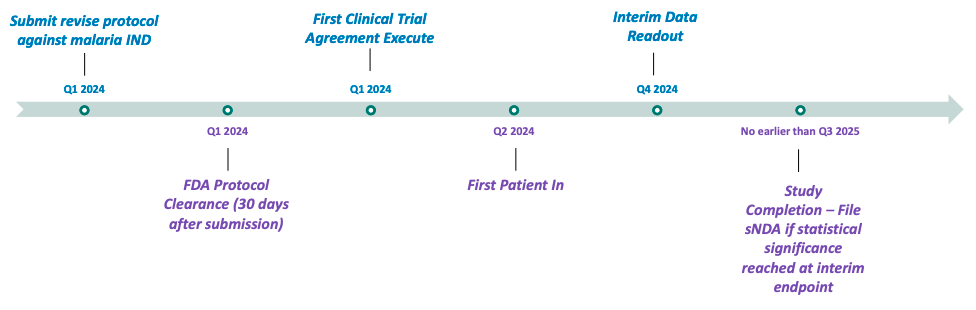
Other Anticipated Milestones:
- Detailed TAM Estimates for tick-borne diseases
- Hiring of Chief Commercial Officer
- Trade & Scientific Conferences
- New product development collaborations
Investor Presentation
Investment Highlights
-
ARAKODA® (tafenoquine) – Overview
- FDA-approved safe, long-acting, mechanistically differentiated anti-malarial
- Discovered by and co-developed with U.S. Army
- 1,100+ patient exposures in 8+ published trials
- Commercially available in U.S. via network of major national distributors
- Existing commercial/regulatory infrastructure expected to facilitate cost-effective pathway to new/expanded indications following targeted clinical trials and label changes
-
ARAKODA Regimen of Tafenoquine - Research & Development Agenda
- Company has strong IP for malaria and other indications
- Preparing to launch a pivotal study of tafenoquine in hospitalized babesiosis patients in mid-2024
- 47,000 cases of babesiosis (infections caused by red blood cell parasites similar to malaria that are transmitted by deer tick bites) occur in the US each year, and the incidence rate is increasing
-
60 Degrees Pharmaceuticals – Overview
- Seasoned Management Team and Board
- Profound clinical expertise in tafenoquine and related therapeutics
- Team has together led/managed four clinical trials
- Collectively led multiple pharmaceutical product approvals/ international pharmaceutical product launches
- Collectively led/provided guidance to multiple public & private entities
- Participated in/led multiple public listings
- Buy rating from Ascendiant Capital Markets
About Babesiosis
- Tick-borne disease caused by protozoan parasites of the genus Babesia
- Invades red blood cells, causing:
- Non-specific flu-like symptoms
- Anemia
- Death (1.6% mortality rate in hospitalized patients/10% in those with cardiac complications)
- May be refractory to treatment in immunosuppressed patients
- Associated with chronic post-treatment syndrome
- Common in Mid-West and Northeastern US
- Geographic range expanding and incidence increasing
- Common coinfection with Lyme disease (10% of cases)
Portfolio
Sign Up For SXTP Email News Alerts
Disclosure
RedChip Companies, Inc. research reports, company profiles, and other investor relations materials, publications or presentations, including web content, are based on data obtained from sources we believe to be reliable but are not guaranteed as to accuracy and are not purported to be complete. As such, the information should not be construed as advice designed to meet the particular investment needs of any investor. Any opinions expressed in RedChip reports, company profiles, or other investor relations materials and presentations are subject to change. RedChip Companies and its affiliates may buy and sell shares of securities or options of the issuers mentioned on this website at any time.
RedChip Visibility is a division of RedChip Companies, Inc. and offers research services to paying clients. In the purview of Section 17(b) of the Securities Act of 1933 and in the interest of full disclosure, we call the reader's attention to the fact that RedChip Companies Inc. is an investor relations firm hired by certain companies to increase investor awareness to the small-cap equity community.
Stock market investing is inherently risky. RedChip Companies is not responsible for any gains or losses that result from the opinions expressed on this website, in its research reports, company profiles, or in other investor relations materials or presentations that it publishes electronically or in print.
We strongly encourage all investors to conduct their own research before making any investment decision. For more information on stock market investing, visit the Securities and Exchange Commission ("SEC") at www.sec.gov and/or the Ontario Securities Commission (“OSC”) at www.osc.gov.on.ca.
Sixty Degrees Pharma (SXTP) is a client of RedChip Companies. SXTP agreed to pay RedChip Companies, Inc. a $9,500 monthly cash fee, beginning in March 2023, and $40,000 of Rule 144 stock (based on the 30-day average price after the IPO) for six months of investor awareness services. SXTP also agreed to pay RedChip a one-time $70,000 fee for a national TV ad campaign aired weekdays from May 20 to June 7, 2024. RedChip receives additional compensation in connection with other investor awareness services for SXTP. For specific details, please visit https://www.redchip.com/legal/disclosures. This is not a solicitation to buy or sell securities.
Investor awareness services and programs are designed to help small-cap companies communicate their investment characteristics. RedChip investor awareness services include the preparation of a research profile(s), multimedia marketing, and other awareness services.
