
bioAffinity Technologies
$1.34 -0.1000 -6.94% 238.9K
Detecting lung cancer early with advanced flow cytometry

Company Overview
bioAffinity Technologies addresses the urgent need for noninvasive, accurate early-stage cancer diagnosis and broad-spectrum cancer treatments. Lung cancer is the leading cause of cancer-related deaths. The Company’s first product, CyPath® Lung, improves early-stage detection of lung cancer, leading to increased survival, fewer unnecessary invasive procedures, reduced patient anxiety, and lower medical costs. CyPath® Lung, a laboratory developed test (LTD), is patient-friendly and physician focused. Physicians order CyPath® Lung for their patients after lung cancer screening reveals a suspicious finding.
Patients collect their sample at home and ship overnight in a pre-paid envelope to the Company’s commercial laboratory, Precision Pathology Laboratory Services, where data is collected using flow cytometry. Proprietary automated data analysis, developed using artificial intelligence (AI), detects lung cancer by analyzing the lung microenvironment to identify cell populations that indicate malignancy. CyPath® Lung has shown high sensitivity (92%), high specificity (87%) and high accuracy (88%) in detecting early-stage lung cancer in patients with small pulmonary nodules less than 20 millimeters. Research and optimization of the Company’s platform technologies are conducted in its laboratories at Precision Pathology and The University of Texas at San Antonio.
Value Proposition
bioAffinity Technologies is an emerging player in early-stage cancer diagnosis and treatment. Its flagship product, CyPath® Lung, improves detection of early-stage lung cancer by analyzing the lung microenvironment. The noninvasive test has shown high sensitivity, specificity and accuracy. Lung cancer often goes undetected until it reaches late-stage when treatment options are less effective. Early detection increases survival and reduces medical costs. With commercialization underway, including a pilot program in Texas and the US Department of Defense’s purchase of tests for ongoing research, the Company is well-poised for growth. The lung cancer diagnostics market is projected to reach $4.7 billion by 2030. The Company’s flow cytometry platform, enhanced by AI-driven automated data analysis, is being developed to aid in noninvasive diagnosis of COPD and asthma, which have a combined global market expected to reach $8.2 billion by 2027. The Company holds extensive U.S. and international patents. Insiders show strong support for the Company with ownership at 35%. With a highly experienced leadership team, bioAffinity Technologies is well-positioned to drive innovation in early-stage cancer diagnosis and treatment.
Hear How Physicians Are Using CyPath® Lung
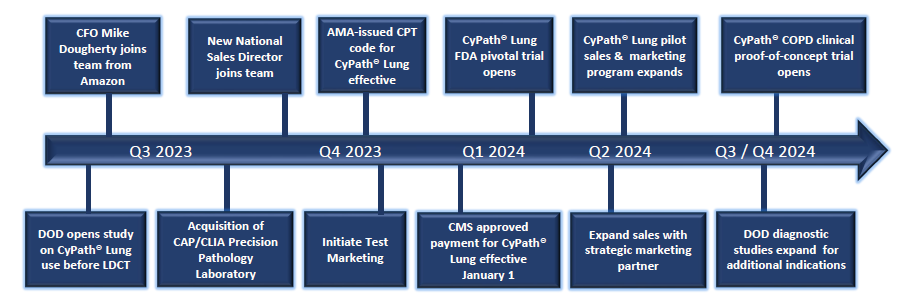
Recent & Anticipated Milestones
Investor Presentation
Investment Highlights
Commercialization of CyPath® Lung test underway
- Expanding sales of CyPath® Lung in beyond initial pilot market in Texas
- US Department of Defense purchased CyPath® Lung for an observational study and research into the test’s use for screening
- AMA issued a CPT code for CyPath® Lung, paving the way for reimbursement by both private payors and public health insurance programs; CMS approved payment became effective January 1, 2024
- 547% annualized growth rate for CyPath® Lung orders in first four months of 2024 over full-year 2023
Lung cancer is the leading cause of cancer-related deaths worldwide
- Lung cancer is frequently detected in advanced stages, resulting in limited treatment options
- Global lung cancer diagnostics market forecast to reach $4.7B by 2030
- Company holds strong international patent portfolio supporting worldwide commercialization
Company’s CAP/CLIA laboratory forecasting between $9.2 million and $9.6 million revenue for 2024, up 23% over 2023
- Company captures 100% of CyPath Lung revenues
- Precision Pathology Laboratory Services provides capacity for nationwide expansion under one structure
- Accretive acquisition completed in 2023; 100% return on cash investment expected in 24 months
Experienced leadership team
- CEO Maria Zannes (30+ years executive-level experience)
- Chief Science & Medical Officer Dr. Vivienne Rebel (20+ years of scientific research experience)
Physician-Focused, Patient-Friendly Market Opportunity

Sign Up For BIAF Email News Alerts
Disclosure
RedChip Companies, Inc. research reports, company profiles, and other investor relations materials, publications or presentations, including web content, are based on data obtained from sources we believe to be reliable but are not guaranteed as to accuracy and are not purported to be complete. As such, the information should not be construed as advice designed to meet the particular investment needs of any investor. Any opinions expressed in RedChip reports, company profiles, or other investor relations materials and presentations are subject to change. RedChip Companies and its affiliates may buy and sell shares of securities or options of the issuers mentioned on this website at any time.
RedChip Visibility is a division of RedChip Companies, Inc. and offers research services to paying clients. In the purview of Section 17(b) of the Securities Act of 1933 and in the interest of full disclosure, we call the reader's attention to the fact that RedChip Companies Inc. is an investor relations firm hired by certain companies to increase investor awareness to the small-cap equity community.
Stock market investing is inherently risky. RedChip Companies is not responsible for any gains or losses that result from the opinions expressed on this website, in its research reports, company profiles, or in other investor relations materials or presentations that it publishes electronically or in print.
We strongly encourage all investors to conduct their own research before making any investment decision. For more information on stock market investing, visit the Securities and Exchange Commission ("SEC") at www.sec.gov and/or the Ontario Securities Commission (“OSC”) at www.osc.gov.on.ca.
bioAffinity (BIAF) is a client of RedChip Companies, Inc. BIAF agreed to pay RedChip Companies, Inc. an $8,500 monthly cash fee, beginning in October 2023 , and 50,000 shares of Rule 144 stock (subject to board approval) for 12 months of investor awareness services. BIAF also agreed to pay RedChip a $100,000 fee for national TV ad campaigns aired February 21 to March 5, 2024, April 1 to April 12, 2024, May 16 to June 7, 2024, June 27 to July 12, 2024, and July 22 to July 26, 2024, and a $30,000 fee for a national TV ad campaign aired September 12 to October 8, 2024.
Investor awareness services and programs are designed to help small-cap companies communicate their investment characteristics. RedChip investor awareness services include the preparation of a research profile(s), multimedia marketing, and other awareness services.
