$0.42 -0.01 ( -1.22% ) 269.4K
NASDAQ: SNGX
Company Overview
Soligenix is a late-stage biopharmaceutical company specializing in developing and commercializing products to treat rare diseases with unmet medical needs. The Company's primary focus is on its Specialized BioTherapeutics business segment, which is responsible for the development of HyBryte™, a novel photodynamic therapy that uses safe visible light for the treatment of cutaneous T-cell lymphoma (CTCL). The Company has successfully completed a Phase 3 study for HyBryte™ and is now negotiating a confirmatory Phase 3 pivotal study design with the US Food & Drug Administration, while also evaluating/pursuing potential HyBryte™ marketing approval ex-U.S. with the successfully completed Phase 3 study.
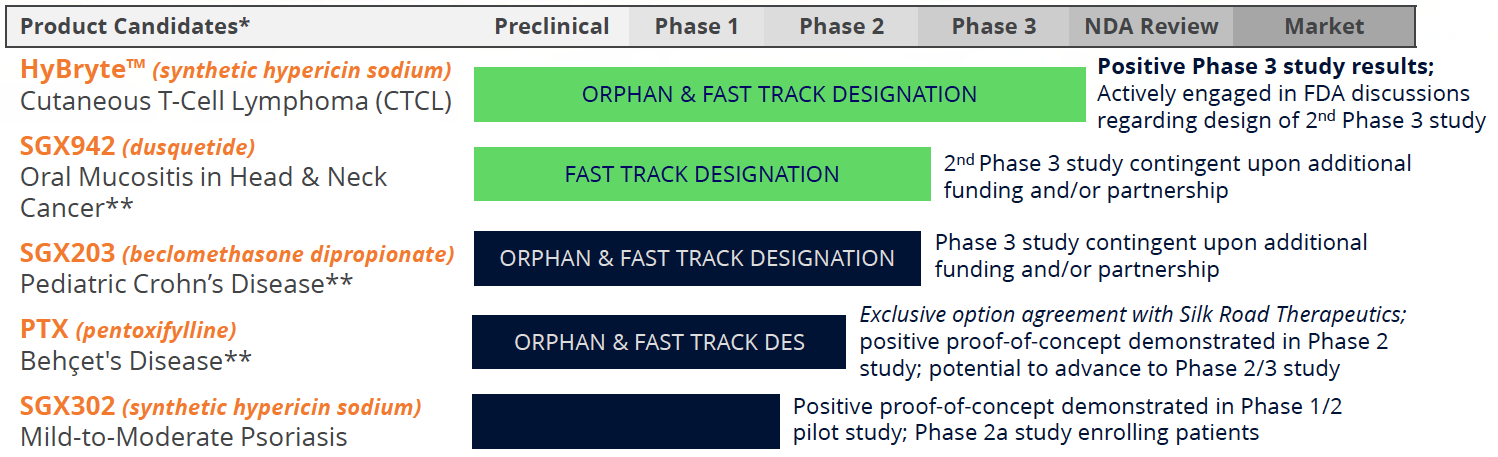
Additionally, Soligenix is expanding synthetic hypericin (SGX302) into psoriasis, as well as developing proprietary formulations of oral beclomethasone 17,21-dipropionate (BDP) for the prevention and treatment of gastrointestinal (GI) disorders characterized by severe inflammation. The Company is also developing a first-in-class innate defense regulator (IDR) technology, dusquetide (SGX942), to treat inflammatory diseases, including oral mucositis in head and neck cancer. Soligenix received FDA IND clearance in Q4 2023 for a Phase 2 trial of dusquetide for the treatment of aphthous ulcers of Behçet's disease.
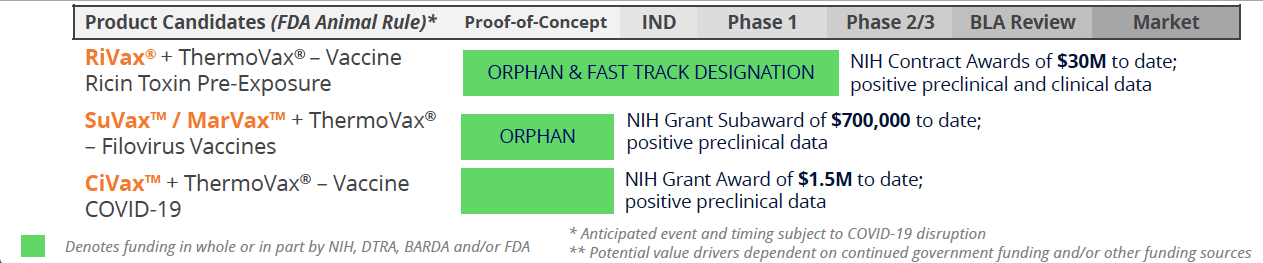
Soligenix's Public Health Solutions business segment is responsible for the development of a number of exciting new products designed to combat a variety of severe medical conditions, including RiVax®, a ricin toxin vaccine candidate that will combat ricin poisoning, and SGX943, a therapeutic candidate designed to combat antibiotic-resistant and emerging infectious diseases. The Company is also working on a number of vaccine programs focused on filoviruses, including Ebola and Marburg, as well as a promising vaccine candidate for the prevention of COVID-19. Soligenix is utilizing a proprietary heat stabilization platform called ThermoVax® to develop its vaccine products. To date, this business segment has been supported with government grant and contract funding from the National Institute of Allergy and Infectious Diseases (NIAID), the Defense Threat Reduction Agency (DTRA), and the Biomedical Advanced Research and Development Authority (BARDA).
Specialized Biotherapeutics Pipeline
Commercial Targets: Unmet Medical Needs in Oncology and Inflammation
Public Health Solutions Pipeline
Funded by Government: Medical Countermeasures for Civilian & Military Use
Value Proposition
Soligenix, a late-stage biopharmaceutical company, offers a robust pipeline of multiple fast-track and orphan-designated products with the potential for significant commercial returns of approximately $2 billion in global annual sales. Soligenix's late-stage clinical assets include HyBryteTM or SGX301, a photodynamic therapy for the treatment of cutaneous T-cell lymphoma (CTCL). The positive statistically significant results achieved in the Phase 3 study of HyBryte have been published in JAMA Dermatology, and the Company is preparing to meet with the FDA to discuss the design of the second confirmatory Phase 3 study. The estimated global market potential for HyBryte is $250 million, making it a significant commercial opportunity in an area of unmet medical need.
Soligenix has also received positive and statistically significant results for its synthetic hypericin (SGX302) in a Phase 1/2 proof-of-concept study for the treatment of psoriasis. The company is now enrolling patients for the Phase 2a study in mild-to-moderate psoriasis. With an estimated global market potential of over $1 billion, SGX302 is another significant commercial opportunity in an area of unmet medical need. The Company is also developing SGX203 for the treatment of pediatric Crohn’s disease, with a pivotal Phase 3 study expected to commence subject to additional funding or partnership.
In addition to its late-stage biotherapeutic assets, Soligenix has established collaborations with biotech, academia, and government agencies for the development of its public health solutions pipeline and has secured non-dilutive government funding to cover operating expenses, including NIH grant awards that support vaccine development and potential for up to three Priority Review Vouchers (PRVs). In January 2024, the Company announced publication of data from its thermostabilized filovirus vaccine program. The data, published in Vaccine, demonstrated complete protection against filovirus disease in nonhuman primate models of Sudan ebolavirus (SUDV) and Marburg marburgvirus (MARV) infections.
In conclusion, Soligenix is a compelling investment opportunity with a promising product pipeline that caters to unmet medical needs. The company’s late-stage clinical assets, ongoing collaborations, non-dilutive funding, and experienced management team position the Company for significant returns.
Market Data
Investment Highlights
- Robust pipeline consisting of multiple fast track and/or orphan designated products, with potential for significant commercial returns of ~$2B in global annual sales
-
Late-stage clinical assets, one with successful Phase 3 data readout
- Cutaneous T-cell lymphoma (HyBryte™ or SGX301)
- Positive statistically significant results achieved in first Phase 3 study; published JAMA Dermatology
- Discussions underway with the FDA for the design of second confirmatory Phase 3 study
- Significant commercial opportunity in area of unmet medical need; estimated global market potential $250M
- Psoriasis (SGX302)
- Positive and statistically significant results achieved in Phase 1/2 proof of concept study
- Expanded Phase 2a study in mild-to-moderate psoriasis enrolling patients after demonstration of biological effect in all five of the initial subjects
- Significant commercial opportunity in area of unmet medical need; estimated global market potential >$1B
- Pediatric Crohn’s disease (SGX203)
- Pivotal Phase 3 study initiation contingent upon additional funding and/or partnership
- Aphthous Ulcers in Behçet's Disease (SGX945)
- FDA IND and Phase 2a protocol clearance received in Q4 2023
- Estimated global market potential >$200M
- Cutaneous T-cell lymphoma (HyBryte™ or SGX301)
- Collaborations with biotech, academia and government agencies
-
Non-dilutive government funding helps cover operating expenses
- NIH grant awards supporting vaccine development; potential for up to 3 Priority Review Vouchers (PRVs)
- Experienced management team and renowned advisors with record of success
Archived Webinar
RedChip Investor Group Call with Soligenix (NASDAQ: SNGX)
Wednesday, February 21, 2024





