$2.99 +0.0200 ( +0.67% ) 116.2K
NASDAQ: RVPH
Company Overview
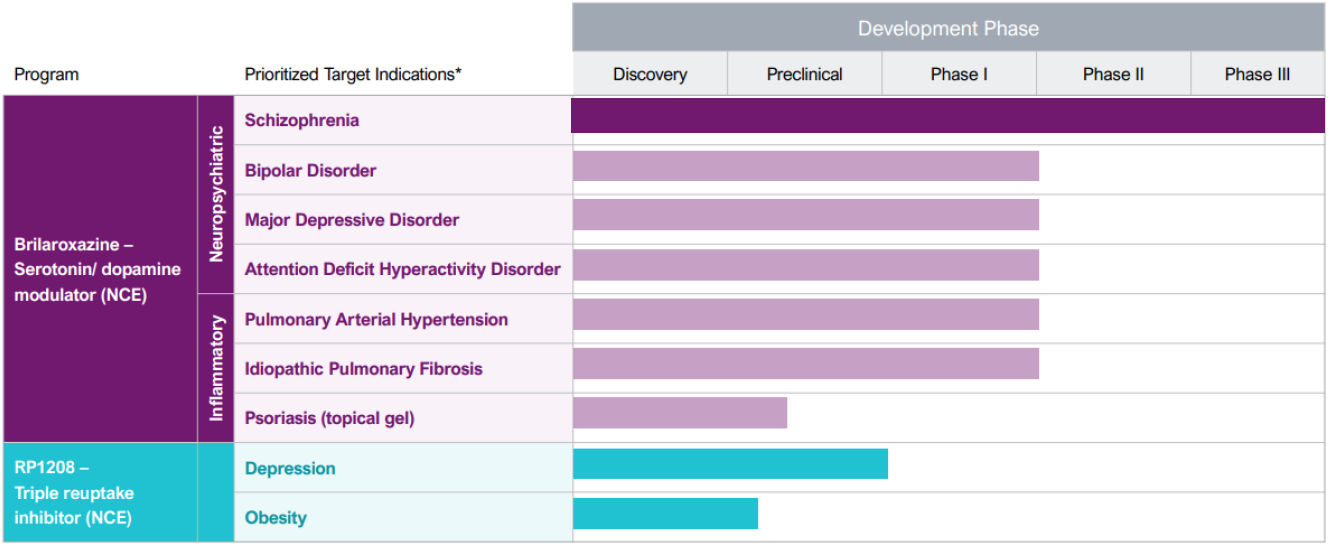
Reviva is a Phase 3 clinical-stage biopharmaceutical company that discovers, develops and seeks to commercialize next-generation therapeutics for diseases representing unmet medical needs and burdens to society, patients, and their families. Reviva's current pipeline focuses on the central nervous system, respiratory and metabolic diseases. Reviva's pipeline currently has two drug candidates, RP5063 (Brilaroxazine) and RP1208. Both are new chemical entities discovered in-house. Positive topline data for pivotal Phase 3 trial evaluating brilaroxazine for the treatment of schizophrenia was reported in Q3 2023. Reviva has been granted composition of matter patents for both RP5063 and R1208 in the US, Europe, and several other countries.
Value Proposition
Reviva is a well-funded Phase 3 biopharma with two in-house discovered platform drug candidates in its pipeline: RP5063, novel serotonin and dopamine receptor modulator, and RP1208, a novel triple reuptake inhibitor. Reviva recently completed its Phase 3 trial of brilaroxazine for the treatment of schizophrenia. The trial successfully met its primary endpoint, with brilaroxazine at the 50 mg dose achieving a statistically significant and clinically meaningful 10.1-point reduction in Positive and Negative Syndrome Scale (PANSS) total score compared to placebo (-23.9 brilaroxazine 50 mg vs. -13.8 placebo, p<0.001) at week 4. Brilaroxazine also achieved statistically significant and clinically meaningful reductions in all major symptom domains and secondary endpoints at week 4 with the 50 mg dose vs. placebo. Reviva plans to continue the clinical development of RP5063 for the treatment of other neuropsychiatric diseases including bipolar, major depressive disorder, ADHD, and psychosis in Alzheimer’s and Parkinson’s diseases. Reviva believes RP1208 is ready to begin IND enabling studies for depression and animal efficacy studies for obesity, following the receipt of adequate additional financing. Five analysts cover Reviva with buy ratings and a mean price target of $16.83 per share. Additionally, the US FDA has granted orphan designation to RP5063 for the treatment of PAH and IPF.
Addressing Significant Unmet Medical Needs
Market Data
Investment Highlights
- Clinical-stage pharmaceutical company developing therapies for central nervous system, cardiovascular, metabolic, and inflammatory diseases
-
Extensive clinical pipeline with recently completed Phase 3 program in schizophrenia
- Phase 3 trial of brilaroxazine met primary and secondary endpoints
- Phase 3 data further confirmed the well-tolerated safety profile
- Brilaroxazine, a serotonin-dopamine signaling modulator, has potential to improve additional key disease drivers like neuroinflammation
- Orphan Drug Designation for the treatment of pulmonary arterial hypertension (PAH) & idiopathic pulmonary fibrosis (IPF)
-
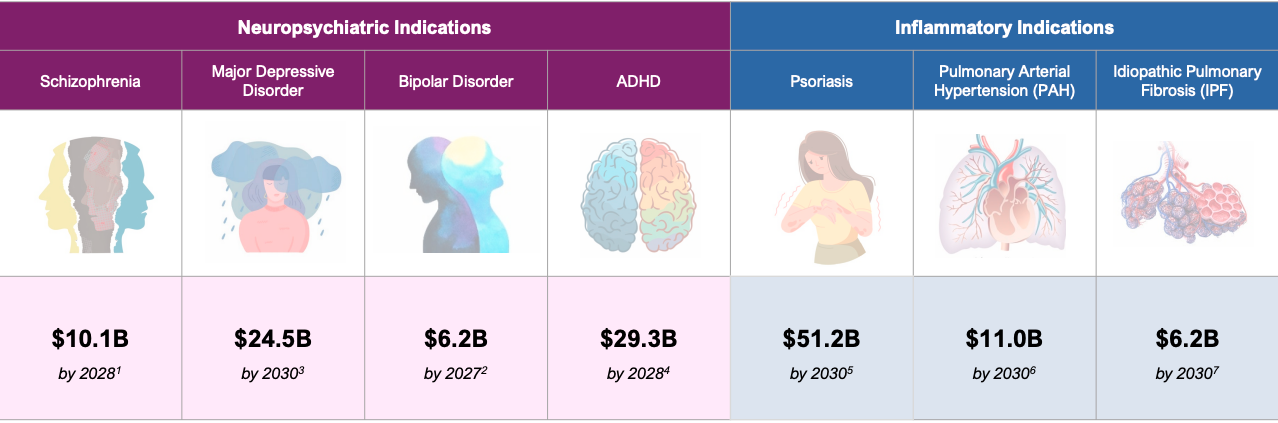
Addressable market opportunities:
- $10.1 billion for schizophrenia by 2028
- $24.5 billion for major depressive disorder by 2030
- $6.2 billion for bipolar disorder by 2028
- $29.3 billion for ADHD by 2025
- $51.2 billion for psoriasis by 2030
- $11.0 billion for PAH by 2030
- $6.2 billion for IPF by 2030
- Approx. $5 million cash on hand as of September 30, 2023; completed registered direct offering in November 2023 raising $30 million gross proceeds
- Strong management team with proven track record in biotech and pharma development
- Five analysts cover Reviva with an average price target of $16.83 per share
Archived Webinar
RedChip Investor Group Call with Reviva Pharmaceuticals Holdings (NASDAQ: RVPH)
Wednesday, December 20, 2023




