BIOLIFE4D Corp.
Private
Company Overview
BIOLIFE4D Corp. is a pioneering, development stage biotech company that plans to leverage current advances in life sciences and cardiac tissue engineering to build human hearts first for cardiotoxicity testing and then potentially for suitable implantation.
We plan to strategically position ourselves at the center of an unprecedented convergence of regenerative medicine, stem cell biology, additive manufacturing (3D printing), and computing technology – all having reached a level of maturity where we believe that commercially viable bioprinting solutions can be created through optimization, not invention. With BIOLIFE4D, a patient-specific, fully functioning heart may be created through 3D bioprinting using the patient’s own cells, potentially eliminating the challenges of organ rejection and long donor waiting lists that plague existing organ transplant methods.
BIOLIFE4D is committed to perfecting the technology to make viable organ repair and replacement an accessible and affordable reality.

Addressable Markets:
Each milestone in BIOLIFE4D’s proposed pipeline represents a unique commercialization opportunity completely independent of each other. Estimated markets which our technology may potentially address include:
Value Proposition
BIOLIFE4D has a robust proposed product pipeline which includes cardiac patches, vascular grafts, heart valves, and a 3D bioprinted human heart. The Company expects to commercialize its first product, the Mini-Heart, in late 2023/early 2024, targeting clinical research organizations and big pharma. The 3D bioprinted Mini-Heart is based on the geometry of a human heart and is being developed for potential use in cardiotoxicity testing in drug and vaccine development. Cardiotoxicity is frequently a major factor in failed human trials. The Mini-Heart potentially offers researchers a way to test drug candidates on a heart that is similar in form and function to a human heart, potentially providing a better predictive model than animal testing used for clinical trials, which could ultimately drive time and cost savings in the discovery phase for drug developers. No FDA approval is currently required to commercialize the Mini-Heart.
Ultimately, BIOLIFE4D is working toward the development of a patient-specific, fully functioning heart created through 3D bioprinting that uses a patient’s own cells, which could potentially address the lack of supply of donor organs and could also improve some of the challenges associated with existing transplant methods, which include organ rejection and the need for extensive immunosuppressant therapy.
As BIOLIFE4D executes on its vision of perfecting the technology to make viable organ replacement an accessible and affordable reality, it is also working toward commercializing bioengineered cardiac components, such as cardiac valves and patches, that the Company believes will meaningfully impact patients with cardiovascular disease while BIOLIFE4D researchers continue development of a full-sized bioengineered human heart.
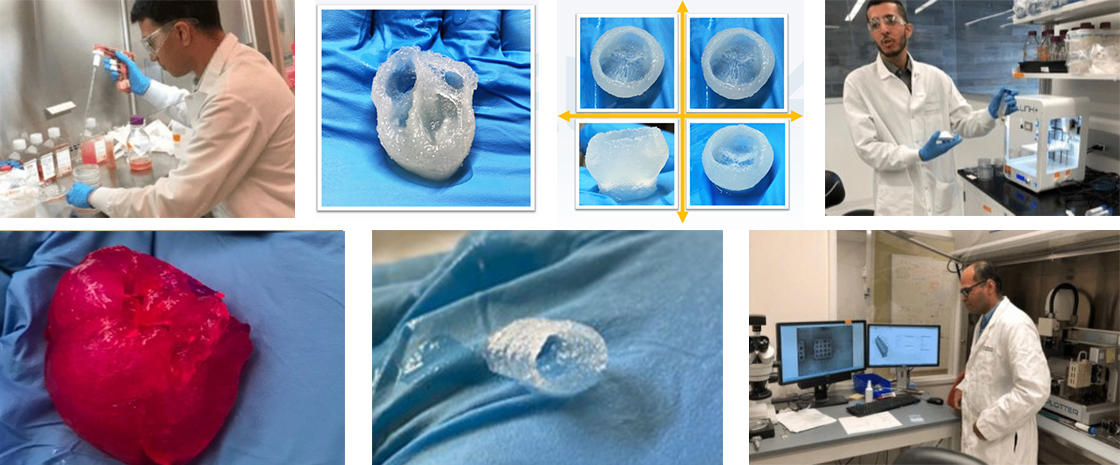
World-Class Strategic Partnerships
Strategic partnerships provide BIOLIFE4D access to and use of their core research facilities, equipment, and personnel expertise.

Investment Highlights
-
Potentially addressing unmet needs in cardiovascular disease
- Cardiovascular disease is the leading cause of death globally
- Due to lack of viable donor organs, only ~3500 heart transplants take place in the U.S. and ~5000 globally each year
- 2030 global cost of cardiac disease is set to rise to $818 billion
-
Potential commercialization of human Mini-Heart in late 2023/2024
- $5B+ market opportunity
- Potentially provide a better predictive model than animal testing used for clinical trials; substantially reducing reliance on animal testing in pharma R&D
- No FDA approval currently required for initial commercialization of the Mini-Heart
-
Developing fully functioning 3D bioprinted heart
- Potential to make viable organ repair and replacement an accessible and affordable reality
- Development path includes multiple milestones that each represent multiple independent market opportunities (cardiac patches; vascular grafts; heart valves)
-
World-class leadership and scientific team collaborating with world-class strategic partners
- BIOLIFE4D utilizes Johnson & Johnson Innovation - JLABS, a premier life science incubator program
- Team with deep experience in life-sciences, biomedical engineering, tissue engineering, and transplantation
Management Team

Steven Morris
CEO, Founder
Steven Morris, possesses extensive research in regenerative medicine — specifically, the medical applications of 3D bioprinting—combined with 15-plus years of hands-on experience in the medical field. Steven brings a wealth of key relationships with suppliers and customers cultivated throughout his many years in business, and has the company well positioned for substantial growth.

Kate Lewis
President
Kate Lewis, A seasoned executive with over 25 years of experience, working alongside global leaders within the pharmaceutical and regulatory industries. Lewis is developing BIOLIFE4D’s message, identifying critical stakeholders, building strategic partnerships, and expanding marketplace position.

Dr. Jeffrey Morgan
CMO
Dr. Jeffrey Morgan currently Surgical Director for Mechanical Circulatory Support and Heart Transplant at the famed Sheba Medical Center in Tel Aviv, Israel. Completed his General Surgery Residency at Mount Sinai Medical Center in New York and his Cardiothoracic Surgery Residency at New York University.

