| Can-Fite (NYSE American: CANF) Announces Final Data Analysis from Phase 2 NASH Study |
| |
|
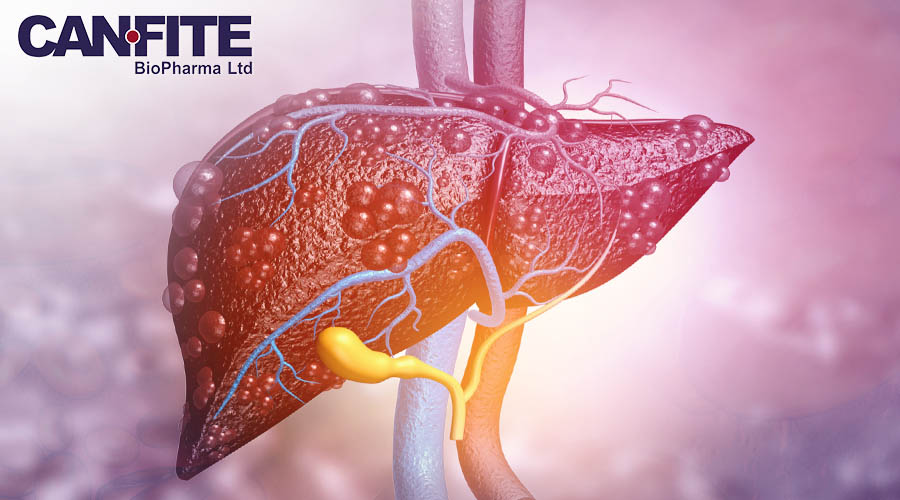
Can-Fite BioPharma (NYSE American: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address inflammatory, cancer and liver diseases, announced that the final data analysis from its Phase 2 study of Namodenoson in the treatment of patients with non-alcoholic fatty liver disease (NAFLD) with or without non-alcoholic steatohepatitis (NASH) has been completed. As a whole, the data show that Namodenoson at the 25 mg dose produced statistically significant results in all measures of efficacy, while also having a strong safety profile and well tolerated.
Why It Matters: New data resulting from the final analysis included additional results from MRI-PDFF (proton density fat fraction on magnetic resonance imaging) and liver stiffness measured by CAP Fibroscan. An evaluation based on per patient liver volume revealed that liver fat volume decreased in the Namodenoson treated groups vs. the placebo (12.5 mg=81.2; 25 mg =102.1, vs. placebo= 33.0) with a high significance (12.5mg p=0.036; 25mg p=0.027, respectively). The percentage of fat volume decrease was also statistically significant, with the Namodenoson 12.5 mg group declining by 3.68%, and the 25 mg group declining by 4.33% vs. the placebo at 2.61% (25 mg p=0.036). This provides additional support for the anti-steatotic effect of Namodenoson and additional assurance that 25 mg is the more efficacious dose.
Key Quote: “We are very pleased with these compelling data. The next clinical trial protocol to advance Namodenoson in the treatment of NASH and NAFLD is now being developed. With the clear need for approved drugs in this indication, I believe distribution partners for Can-Fite will likely take notice of these results.” – Prof. Rifaat Safadi, Principal Investigator
The Backstory: Can-Fite is an advanced clinical stage drug development Company with a platform technology that is designed to address multi-billion dollar markets in the treatment of cancer, inflammatory disease and COVID-19. The Company's lead drug candidate, Piclidenoson, is currently in Phase III trials for rheumatoid arthritis and psoriasis. Piclidenoson has been approved for a pilot clinical trial in Israel to treat COVID-19 infected patients with moderate-to-severe symptoms. Can-Fite's liver drug, Namodenoson, is headed into a Phase III trial for hepatocellular carcinoma (HCC), the most common form of liver cancer, and successfully achieved its primary endpoint in a Phase II trial for the treatment of non-alcoholic steatohepatitis (NASH). Namodenoson has been granted Orphan Drug Designation in the U.S. and Europe and Fast Track Designation as a second line treatment for HCC by the U.S. Food and Drug Administration. Namodenoson has also shown proof of concept to potentially treat other cancers including colon, prostate, and melanoma. CF602, the Company's third drug candidate, has shown efficacy in the treatment of erectile dysfunction. These drugs have an excellent safety profile with experience in over 1,500 patients in clinical studies to date.
Disclosure
Can-Fite Biopharma (CANF) is a client of RedChip Companies, Inc. CANF agreed to pay RedChip Companies, Inc. a cash fee of $5,000 monthly, beginning in August 2019, and 16,500 shares of CANF Rule 144 stock for 6 months of RedChip investor awareness services and consulting services.
|
|
| |
|
| Recruiter.com (NASDAQ: RCRT) Closes Oversubscribed Convertible Debenture Bridge Financing |
| |
|
Recruiter.com (NASDAQ: RCRT), a leading hiring platform for the world's largest network of recruiters, announced that it has successfully closed an oversubscribed private placement of convertible debentures in the aggregate principal amount of approximately $2,953,000 bearing an annual interest rate of 8%. Both funds and private individual accredited investors participated in the offering.
Why It Matters: The Company intends to direct the proceeds of the financing into programs that engage the Recruiter.com network of over 25,000 small and independent recruiters, sales, and general corporate purposes, including the anticipated financial, administrative, and legal costs for an uplisting to a major market exchange.
Key Quote: “On behalf of all of us at Recruiter.com, I would also like to welcome the new group of investors who participated in this round of financing, and look forward to sharing our successes in the coming months, as we continue to build traction tied to our growth objectives.” – Evan Sohn, CEO
Backstory: Recruiter.com is a hiring platform for the world's largest network of recruiters, empowing businesses to recruit specialized talent faster with virtual teams of recruiters and AI job-matching technology.
Disclosure
Recruiter.com (RCRT) is a client of RedChip Companies, Inc. RCRT agreed to pay RedChip Companies, Inc. 9,000 shares of RCRT common stock and a $7,500 monthly cash fee, beginning in June 2020 for RedChip investor awareness services.
|
|
| |
|
| CytoDyn (OTCQB: CYDY) Signs Agreement with American Regent for US Distribution of Leronlimab for Treatment of COVID-19 |
| |
|
CytoDyn (OTCQB: CYDY), a late-stage biotechnology company developing leronlimab (PRO 140), a CCR5 antagonist with the potential for multiple therapeutic indications, announced today it has signed an exclusive Distribution and Supply Agreement with American Regent for the distribution of leronlimab for the treatment of COVID-19 in the United States.
Why It Matters: CytoDyn is currently enrolling a Phase 2b/3 clinical trial for 390 severe and critically ill COVID-19 patients, which is a randomized, placebo-controlled with 2:1 ratio (active drug to placebo ratio). The Company has also completed its enrollment of a Phase 2 randomized clinical trial with 75 patients in the mild-to-moderate COVID-19 population. CytoDyn has been granted more than sixty emergency Investigational New Drug (eIND) authorizations by the U.S. Food and Drug Administration (FDA) and plans to provide clinical updates for this patient population in the coming weeks.
Key Quote: “Having this distribution agreement in place ahead of the readout from CytoDyn’s COVID-19 clinical trials further emphasizes CytoDyn’s commitment to making leronlimab immediately available to patients based on the successful completion of its ongoing clinical trials.” – Nader Pourhassan, Ph.D., President and CEO
The Backstory: CytoDyn is a late-stage biotechnology company developing innovative treatments for multiple therapeutic indications based on leronlimab, a novel humanized monoclonal antibody targeting the CCR5 receptor. CCR5 appears to play a critical role in the ability of HIV to enter and infect healthy T-cells. The CCR5 receptor also appears to be implicated in tumor metastasis and immune-mediated illnesses, such as GvHD and NASH. CytoDyn has successfully completed a Phase 3 pivotal trial with leronlimab in combination with standard antiretroviral therapies in HIV-infected treatment-experienced patients. CytoDyn filed its BLA in April 2020 to seek FDA approval for leronlimab as a combination therapy for highly treatment experienced HIV patients, and submitted additional FDA requested clinical datasets on May 11, 2020. CytoDyn is also conducting a Phase 3 investigative trial with leronlimab as a once-weekly monotherapy for HIV-infected patients. CytoDyn plans to initiate a registration-directed study of leronlimab monotherapy indication. If successful, it could support a label extension. Clinical results to date from multiple trials have shown that leronlimab can significantly reduce viral burden in people infected with HIV. No drug-related serious site injection reactions reported in about 800 patients treated with leronlimab and no drug-related SAEs reported in patients treated with 700 mg dose of leronlimab. Moreover, a Phase 2b clinical trial demonstrated that leronlimab monotherapy can prevent viral escape in HIV-infected patients; some patients on leronlimab monotherapy have remained virally suppressed for more than five years. CytoDyn is also conducting a Phase 2 trial to evaluate leronlimab for the prevention of GvHD and a Phase 1b/2 clinical trial with leronlimab in metastatic triple-negative breast cancer.
Disclosure
CytoDyn (CYDY) is a client of RedChip Companies, Inc. CYDY agreed to pay RedChip Companies, Inc., a $7,500 quarterly cash fee, beginning in May 2020, for RedChip investor awareness services. The CEO of RedChip Companies owns 3400 shares of CYDY.
|
|
| |
|
| Digital Ally (NASDAQ: DGLY) Announces its Shield Brand of Safety Products to be Co-Primary Sponsor of Spencer Pigot’s Entry for the GMR Grand Prix |
| |
|
Digital Ally (NASDAQ: DGLY), which develops, manufactures and markets advanced video recording products and other critical safety products for law enforcement, emergency management, fleet safety and security, today announced they will feature their newly-launched product line, Shield™ Cleansers, as a co-primary sponsor of the No. 45 entry for Spencer Pigot for the GMR Grand Prix at Indianapolis Motor Speedway.
Why It Matters: The company recently launched two product lines in direct response to the increased safety precautions organizations and individuals are taking due to the Covid-19 pandemic. ThermoVuä was launched as a non-contact temperature-measuring instrument that measures temperature through the wrist and controls entry to facilities when temperature measurements exceed pre-determined parameters. ThermoVuä has optional features such as facial recognition to improve facility security by restricting access based on temperature and/or facial recognition reasons. ThermoVuä provides an instant pass/fail audible tone with its temperature display and controls access to facilities based on such results. It can be widely applied in schools, office buildings, subway stations, airports and other public venues.
Key Quote: “Current events are bringing widespread attention to the importance of body cameras, leading us to launch a much-needed subscription program for first responders. Current events are also bringing about a demand for virus-fighting weapons such as disinfectants and temperature-measurement instruments. Digital Ally is in a great position to answer the call in providing effective and safe products where and when they’re needed the most.” – Stan Ross, CEO
The Backstory: Headquartered in Lenexa, Kansas, Digital Ally specializes in the design and manufacturing of the highest quality video recording equipment and video analytic software. Digital Ally pushes the boundaries of technology in industries such as law enforcement, emergency management, fleet safety and security. Digital Ally’s complete product solutions include vehicle and body cameras, flexible software storage, automatic recording technology and various critical safety products. These products work seamlessly together and are simple to install and operate. Digital Ally products are sold by domestic direct sales representatives and international distributors worldwide.
Disclosure
Digital Ally, Inc. (DGLY) is a client of RedChip Companies, Inc. DGLY agreed to pay RedChip Companies, Inc., a $7,500 monthly cash fee, beginning in September 2017, for RedChip investor awareness services.
|
|
| |
|
| Milestone Scientific (NYSE American: MLSS) Announces Closing of $14.6 Million Offering of Common Stock and Warrants |
| |
|
Milestone Scientific (NYSE American: MLSS), a leading developer of computerized drug delivery instruments that provide painless and precise injections, today announced that on June 30, 2020 it closed its previously announced underwritten offering of 6,520,000 shares of its common stock and warrants to purchase up to an aggregate of 3,260,000 shares of the its common stock.
Details:: Each share of common stock was sold together with a warrant to purchase 0.50 of one share of common stock at a combined price to the public of $2.15. Gross proceeds before underwriting discounts and commissions and estimated offering expenses, were approximately $14.0 million. In addition, Milestone Scientific granted to Maxim Group LLC a 45-day option to purchase up to an additional 978,000 shares of common stock and/or warrants to purchase up to 489,000 shares of common stock for the purposes of covering any over-allotments, at the public offering price less discounts and commissions, of which Maxim Group LLC partially exercised its option to purchase 250,000 shares of common stock and warrants to purchase up to 489,000 shares of common stock. The over-allotment exercise transaction also closed on June 30, 2020, bringing the total gross proceeds of the offering, before underwriting discounts and commissions and estimated offering expenses, to approximately $14.6 million.
The Backstory: Milestone Scientific is a biomedical technology research and development company that patents, designs and develops innovative diagnostic and therapeutic injection technologies and instruments for medical, dental, cosmetic and veterinary applications. Milestone's computer-controlled systems are designed to make injections precise, efficient, and virtually painless. Milestone's proprietary DPS Dynamic Pressure Sensing technology® is its technology platform that advances the development of next-generation devices, regulating flow rate and monitoring pressure from the tip of the needle, through platform extensions for local anesthesia for subcutaneous drug delivery, with specific applications for cosmetic botulinum toxin injections, epidural space identification in regional anesthesia procedures and intra-articular joint injections.
Disclosure
Milestone Scientific (MLSS) is a client of RedChip Companies, Inc. MLSS agreed to pay RedChip Companies, Inc. 18,000 shares of Rule 144 stock for three months of RedChip investor awareness services and consulting services, beginning April 2020.
|
|
| |
|
| Document Security Systems (NYSE American: DSS) Prices $6.43 Million Underwritten Public Offering of Common Stock |
| |
|
Document Security Systems (NYSE American: DSS), a multinational company operating businesses focused on brand protection technology, blockchain security, direct marketing, healthcare, real estate, and securitized digital assets, today announced the pricing of an underwritten public offering of 1,028,800 shares of its common stock at a price of $6.25 per share, for gross proceeds to the Company of $6.43 million, before deducting underwriting discounts and other offering expenses. The Company intends to use the proceeds to fund growth of their new business lines, acquisition opportunities, and general corporate and working capital.
Backstory: DSS is a multinational company operating businesses focused on brand protection technology, blockchain security, direct marketing, healthcare, real estate, and securitized digital assets. Its business model is based on a distribution sharing system in which shareholders will receive shares in its subsidiaries as DSS strategically spins them out into IPOs. Its historic business revolves around counterfeit deterrent and authentication technologies, smart packaging, and consumer product engagement. DSS is led by its Chairman and largest shareholder, Mr. Fai Chan, a highly successful global business veteran of more than 40 years specializing in corporate transformation while managing risk. He has successfully restructured more than 35 corporations with a combined value of $25 billion.
Disclosure
Document Securities Systems (DSS) is a client of RedChip Companies, Inc. DSS agreed to pay RedChip Companies, Inc. 21,000 shares of DSS Rule 144 stock for 14 months of service, beginning June 2020.
|
|
| |
|
| Nemaura Medical (NASDAQ: NMRD) Reports Results and Provides Business Update for the Fiscal Year Ended March 31, 2020 |
| |
|
Nemaura Medical (NASDAQ: NMRD), a medical technology company commercializing sugarBEAT®, a non-invasive and flexible continuous glucose monitor (CGM), together with BEAT®diabetes, a health subscription service designed to help people with diabetes and prediabetes to better manage their condition through personalized lifestyle coaching, today provided a business update and released financial results for the fiscal year ended March 31, 2020.
Key Highlights:
- Received positive feedback from the first users of sugarBEAT® as part of the first phase of commercial launch
- Announced positive initial user-volunteer data from its head-to-head comparison with a major incumbent CGM device
- Set out plans to launch a digital healthcare subscription service in the U.S. under the brand name proBEAT™, targeted at over 25 million people with Type 2 diabetes and 88 million people with pre-diabetes in the U.S.
- Launched its sugarBEAT® app for Android devices in preparation for the planned commercial launch of sugarBEAT®
- Joined the small cap Russell 2000® Index and the broad-market Russell 3000® Index
- Has entered in to multiple verbal non-binding agreements for sugarBEAT® with the aim of securing regional and global partnerships.
- Reported plans for a new product line by seeking to immediately repurpose sugarBEAT® as a Continuous Temperature Monitor (CTM)
- Secured a 24-month, $5 million non-convertible loan to accelerate commercial strategy
- Announced stockholder approval of the Nemaura Medical Inc. 2020 Omnibus Incentive Plan
- Dr. Faz Chowdhury, Nemaura Medical’s CEO, presented at the Credit Suisse 2020 Virtual Diabetes Sessions
- Dr. Fred Schaebsdeu, Nemaura Medical’s VP of Strategy & Strategic Alliances participated as a guest speaker at the DiabetesMine™ D-Data Virtual ExChange
Backstory: Nemaura Medical is a medical technology company developing micro-systems-based wearable diagnostic devices and currently commercializing sugarBEAT®, and proBEAT™. sugarBEAT®, a CE mark approved Class IIb medical device, is a non-invasive and flexible CGM providing actionable insights derived from real time glucose measurements and daily glucose trend data, which may help people with diabetes and pre-diabetes to better manage, reverse and prevent the onset of diabetes. Nemaura is planning to submit a PMA application for sugarBEAT® during the second quarter of 2020 for FDA review of this device under medical device regulations. proBEAT™ comprises a non-invasive glucose monitor and a digital healthcare subscription service and is due to be launched in the US as a general wellness product.
Disclosure
Nemaura Medical (NMRD) is a client of RedChip Companies. NMRD agreed to pay RedChip a monthly cash fee of $6,000 and 15,000 shares of Rule 144 stock for three months of investor awareness services.
|
|
| |
|
| Watch Replay of Nemaura Medical (NASDAQ: NMRD) CEO in Investor Webinar and Q&A Session from July 7, 2020 |
| |
|
Nemaura Medical (NASDAQ: NMRD), a medical technology company focused on developing micro-systems-based wearable diagnostic devices and currently commercializing sugarBEAT®, its non-invasive and flexible continuous glucose monitor ("CGM"), together with BEAT®diabetes, a planned health subscription service designed to help people with Type 2 diabetes and prediabetes through personalized lifestyle coaching, today announced that its CEO Dr. Faz Chowdhury will participate in an investor webinar, hosted by RedChip Companies, on Tuesday, July 7, 2020 at 11:00 a.m. ET. A live Q&A session with Dr. Chowdhury will follow the presentation.
During the presentation, Dr. Chowdhury will provide an update on the Company’s developments including commercialization progress, US product launch, and status of collaborative partnerships, as well as update on a continuous temperature monitor (CTM) for both the diabetes and non-diabetes markets.
Click here to watch free webinar replay, please visit: www.redchip.com/events/64/nemaura-medical-webinar/nmrd
Disclosure
Nemaura Medical (NMRD) is a client of RedChip Companies. NMRD agreed to pay RedChip a monthly cash fee of $6,000 and 15,000 shares of Rule 144 stock for three months of investor awareness services.
|
|
| |
|
| |
| Quote of the Week |
| |
"Work hard, be a long-term thinker, develop tenacity and remember nothing happens without taking calculated risks. "
- Phil Sassower, CEO of Xplore Technologies |
|
|
|
|
|
|
|
| About RedChip |
| |
| RedChip Companies, an Inc. 5000 company, is an international investor relations, media, and research firm focused on small-cap and mid-cap companies. Since 1992, RedChip has delivered concrete, measurable results for its clients through the most comprehensive service platform in the industry for small-cap and mid-cap companies. These services include a worldwide distribution network for its stock research written by analysts holding the CFA designation; retail and institutional roadshows in major U.S. cities; outbound marketing to stock brokers, RIAs, institutions, and family offices; a digital media investor relations platform that has generated over 2.3 million unique investor views; quarterly global online institutional and retail investor conferences that reach over 10,000 investors annually; "The RedChip Money Report" television show which airs in 100 million homes across the U.S. on The Family Channel; a weekly newsletter delivered to 60,000 investors; TV commercials in local and national markets; corporate and product videos; website design; and traditional investor relation services, which include press release writing, development of investor presentations, quarterly conference call script writing, strategic consulting, capital raising, and more. |
| |
| RedChip Disclosure |
| |
RedChip Companies, Inc. research reports, company profiles and other investor relations materials, publications or presentations, including web content, are based on data obtained from sources we believe to be reliable but are not guaranteed as to accuracy and are not purported to be complete. As such, the information should not be construed as advice designed to meet the particular investment needs of any investor. Any opinions expressed in RedChip reports, company profiles, or other investor relations materials and presentations are subject to change. RedChip Companies and its affiliates may buy and sell shares of securities or options of the issuers mentioned on this website at any time.
RedChip Visibility is a division of RedChip Companies, Inc. and offers research services to paying clients. In the purview of Section 17(b) of the Securities Act of 1933 and in the interest of full disclosure, we call the reader's attention to the fact that the RedChip Companies Inc. is an investor relations firm hired by certain Companies to increase investor awareness to the small-cap equity community.
Stock market investing is inherently risky. RedChip Companies is not responsible for any gains or losses that result from the opinions expressed on this website, in its research reports, company profiles or in other investor relations materials or presentations that it publishes electronically or in print.
We strongly encourage all investors to conduct their own research before making any investment decision. For more information on stock market investing, visit the Securities and Exchange Commission ("SEC") at www.sec.gov and view RedChip’s Disclosures. |
|
| |
|
|
| |
|
|
Copyright © 2020 RedChip Companies, Inc. All Rights Reserved.
You are receiving this message because this email address has signed up to receive News Alerts.
|
|
|
|
|
|