
Date: Thursday, December 12, 2023
Time: 4:15 - 5:00 PM ET
Register
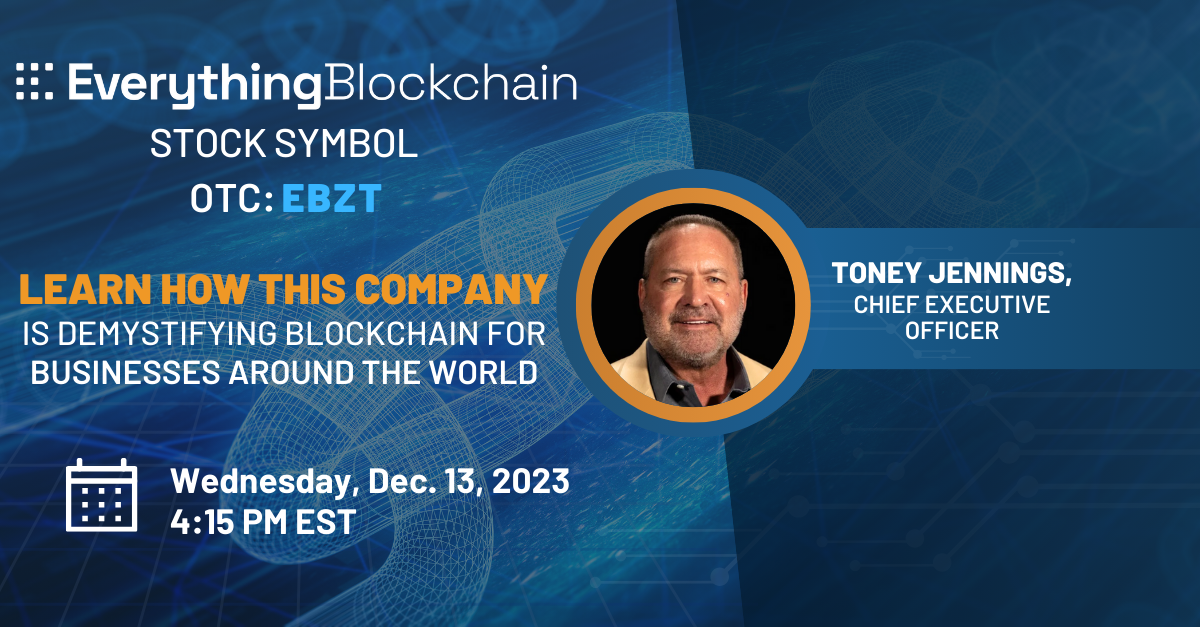
Date: Wednesday, December 13, 2023
Time: 4:15 - 5:00 PM ET
Register

Date: Thursday, December 14, 2023
Time: 4:15 - 5:00 PM ET
Register

Date: Wednesday, December 20, 2023
Time: 4:15 - 5:00 PM ET
Register

Date: Tuesday, January 9, 2024
Time: 4:15 - 5:00 PM ET
Register

Date: Thursday, January 25, 2024
Time: 4:15 - 5:00 PM ET
Register

Date: Thursday, January 11, 2024
Time: 4:15 - 5:00 PM ET
Register

Date: Tuesday, January 16, 2024
Time: 4:15 - 5:00 PM ET
Register

Date: Tuesday, January 23, 2024
Time: 4:15 - 5:00 PM ET
Register

Date: Tuesday, January 30, 2024
Time: 11:00 - 12:00 PM ET
Register

Date: Wednesday, January 24, 2024
Time: 4:15 - 5:00 PM ET
Register

Date: Thursday, February 1, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Thursday, February 8, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Wednesday, February 21, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Tuesday, March 5, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Tuesday, March 19, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Thursday, March 21, 2024
Time: 11:00 AM - 12:00 PM ET
Register

Date: Thursday, March 21, 2024
Time: 4:15 PM - 5:00 PM ET
Register
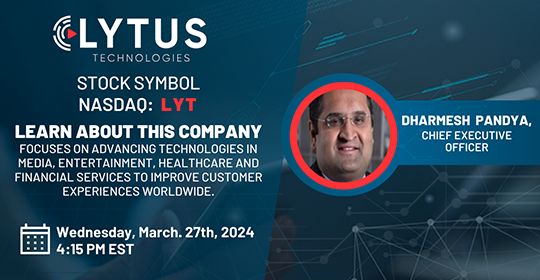
Date: Wednesday, March 27, 2024
Time: 4:15 PM - 5:00 PM ET
Register
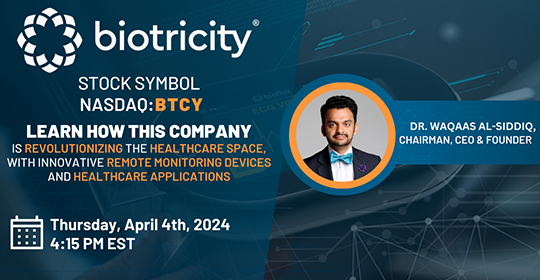
Date: Thursday, April 4, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Tuesday, April 9, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Wednesday, April 10, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Thursday, April 11, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Friday, April 12, 2024
Time: 8:30 AM - 9:30 AM ET
Register

Date: Tuesday, April 16, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Thursday, April 18, 2024
Time: 4:05 PM - 5:00 PM ET
Register

Date: Wednesday, April 17, 2024
Time: 4:15 PM - 5:00 PM ET
Register
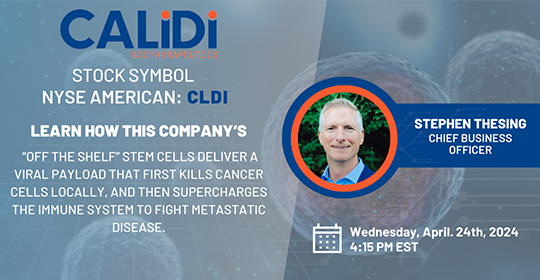
Date: Wednesday, April 24, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Tuesday, April 30, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Thursday, May 2, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Wednesday, May 8, 2024
Time: 4:15 PM - 5:00 PM ET
Register

Date: Thursday, May 16, 2024
Time: 4:15 PM - 5:00 PM ET
Register